Representativeness
Hologenomic analysis of individual animals requires enough genomic and metagenomic DNA to properly characterise host genomes and microbial metagenomes.
Samples should* come from individual captured animals, and host's metadata should also be gathered. *exceptions are possible.
Samples must be frozen within 14 days, and the time and conditions before freezing must be acknowledged.
Samples need to be obtained using gloves and following the best sampling practices guidelines of EHI to minimise human and cross-contamination.
Samples need to be obtained, preserved and shipped following the EHI guidelines. Any deviation must be acknowledged.

The spreadsheet contains information of the field collaborator, the sampling period and the species/sites/individuals that are expected to sample within the mentioned period. These estimations are used to setup a sampling kit tailored to the requirements of the collaborator.
Choose from the large selection of latest pre-made blocks - full-screen intro, bootstrap carousel, content slider, responsive image gallery with lightbox, parallax scrolling, video backgrounds, hamburger menu, sticky header and more.
Sites made with Mobirise are 100% mobile-friendly according the latest Google Test and Google loves those websites (officially)!
Mobirise themes are based on Bootstrap 3 and Bootstrap 4 - most powerful mobile first framework. Now, even if you're not code-savvy, you can be a part of an exciting growing bootstrap community.
Choose from the large selection of latest pre-made blocks - full-screen intro, bootstrap carousel, content slider, responsive image gallery with lightbox, parallax scrolling, video backgrounds, hamburger menu, sticky header and more.
Up to 162 sample collection tubes pre-labelled and pre-filled with preservation buffer.
For sampling the oral cavity and anus of animals. Adjusted to the size of the targeted animals.

Simple guidelines to ensure standardised sample collection and preservation.
Collaborators with limited resources can request complementary materials such as gloves, tweers or face masks.
Hologenomic analysis of individual animals requires enough genomic and metagenomic DNA to properly characterise host genomes and microbial metagenomes.
Not all field researchers have the same capacity to obtain samples from individual animals, due to animal's biological features, fieldwork conditions or sampling permit limitations.
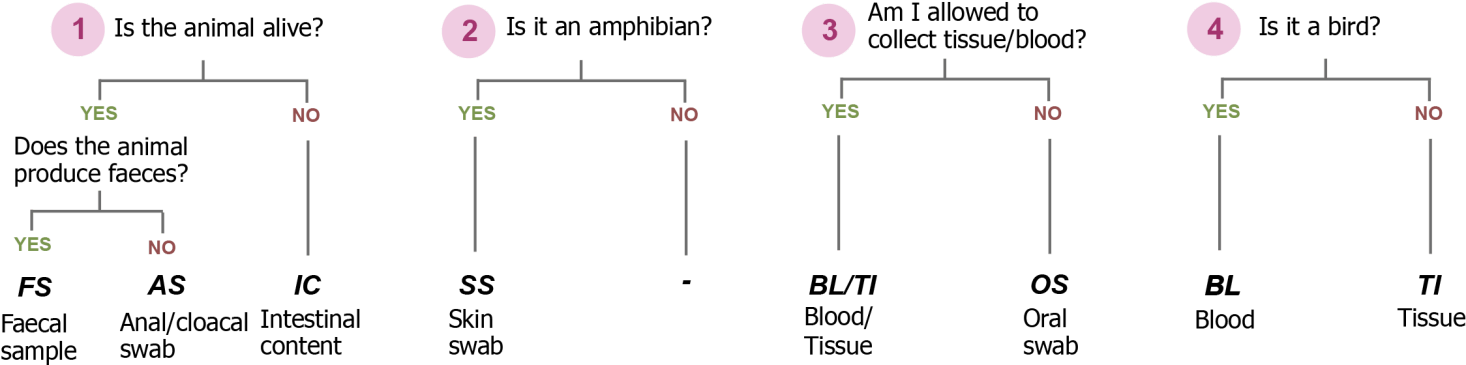
We therefore implement a flexible hierarchical sampling design, that is adjusted to the capacities of field researchers and properties of the samples organisms.
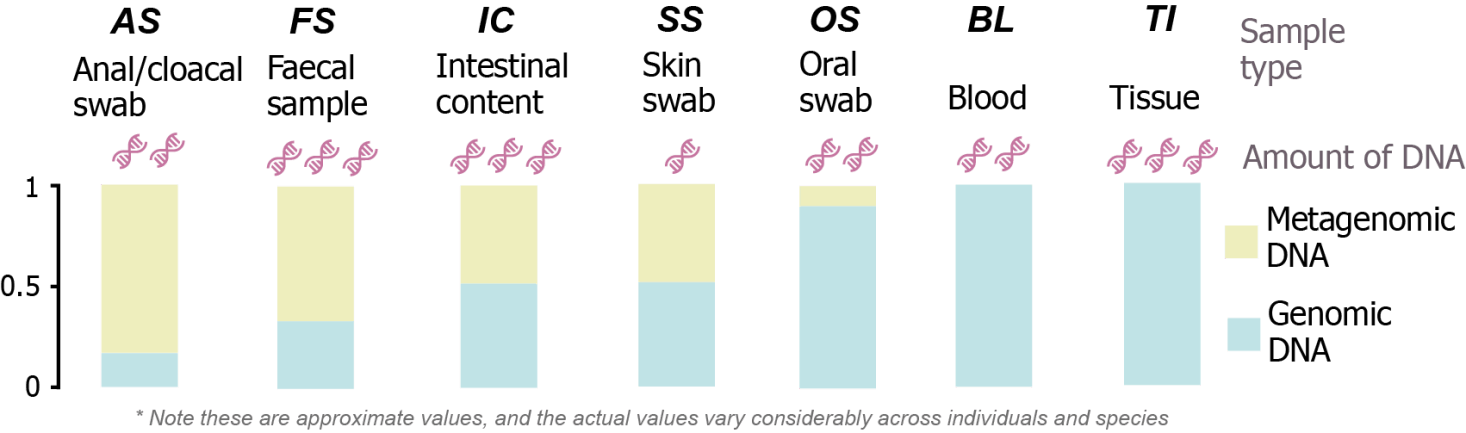
Faeces and anal/cloacal swabs aim to capture the microbial community of the lower intestine. The appropriateness of using one or the other sample type will be determined by the host organisms. Both sample types contain host and microbial DNA, although the ratio between host and microbial DNA can vary drastically across sampled individuals and species. Thus, this sample types might not ensure correct host genotyping, mainly in small animals.
Buccal swabs aim to capture the oral microbial community as well as to provide a larger amount of host DNA for genomic characterisation. When combining with Type 1 samples, there is usually enough DNA to correctly genotype the host animal.
Tissue and blood samples are the best sample types for genomic characterisation of host animals. Obtaining this type of samples will ensure genomic characterisation is properly performed. Tissue samples are preferred in mammals (e.g. ear punch in micromammals, wing punch in bats), amphibians and reptiles, while blood samples are preferred in birds.
Mainly relevant for amphibians. These type of samples enables characterising the skin microbiome of the organisms.
If field researchers are able to freeze the samples immediatelly, they are requested to obtain faecal samples or anal/cloacal/buccal/skin swabs in a buffer (provided by EHI) containing PBS and glycerol to keep microorganisms alive while frozen. This samples will contribute to the wild animal-associated microbial biobank on EHI.
Frequently Asked Questions
How much faecal material should I collect per animal?
Each collection tube contains 1ml of preservation buffer that performs best at 1:10 ratio. Thus, the maximum amount of faecal material should not exceed 100 mg approx. More information can be found in the sampling guidelines.
If possible, should I get both faeces and anal swabs?
It is not necessary to do so with all the samples, but paired faecal and anal swab samples from the same individuals are welcome to 1) assess the differences between both sample types in different organisms, and 2) to increase the sample material. Obtaining both sample types is specially advised when the faecal material is very small (< 20-30 mg).
How much should I insert the swab for anal/cloacal sampling?
It is necessary to insert the cotton part of the swab into the anal or cloacal cavity of the animal in order to sample the microbiome of the lower intestine.
Should I get skin samples from non-amphibian vertebrates?
It is not prioritised, but such skin samples are welcome in some cases. Contact the EHI management team in case of doubt.